Water Disinfection with Ozone
by Rob Dunham, Hong He & Ken Woodard
![]()
Table of Contents

General Information
Van Mauten first reported the existence of ozone in 1785. Schlobein was the
first to isolate it, in 1840. Ozone was used as early as 1893 for disinfection
of drinking water in Holland. Nice, France has employed ozonation of drinking
water since 1906 (Nickols,et al,1992). Europeans used ozone to treat swimming
pools during the 1950s to eliminate the irritated eyes and skin caused by
chlorine. By 1980, there were over 1100 water treatment facilities utilizing
ozone, mostly in Europe (Coate, 1997). Today, more than 2,000 water treatment
plants throughout the world use ozonation for disinfection (Nickols,et
al,1992).
Disinfection practice in the United States has developed principally around
the use of chlorine. Because of concerns about byproduct formation, ozonation
has become an alternative method to avoid the formation of halogenated organics
caused by chlorination (Nickols,et al,1992). Prior to World War II, several
water treatment plants in New York, Pennsylvania, and Indiana experimented with
ozone. As of 1990, there are close to 40 U.S. water treatment plants that are
equipped with ozonation facilities, including the third largest plant in the
world, which is located in Los Angeles. About 40 percent of U.S. ozone plants
use lake water as a source, and the remaining plants are evenly split between
river, reservoir, and groundwater sources (Brink,et al,1991).
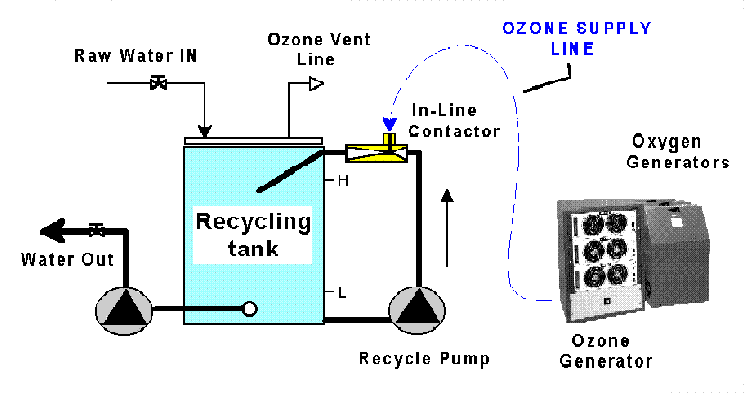 This drawing illustrates a typical ozone treatment
unit. This drawing illustrates a typical ozone treatment
unit.
Drawing courtesy of Ozonair Corporation
Return to
the table of contents

Chemistry
Schlobein named ozone (chemical formula O3) after the Greek word meaning
"smell." Ozone has a very distinctive smell, which is the "fresh and
invigorating" odor (Towles, 1997, a) that can be detected in the air after a
flash of lightning. It has been referred to as "nature's air purifier" (Towles,
1997, a). Ozone is highly unstable, which is why it must be generated on-site at
the water treatment plant.
Ozone (molecular weight 48) is colorless at room temperature and has pungent
odor. It is generally encountered in dilute form in a mixture with oxygen or
air. Liquid O3 is very unstable and will readily explode. Ozone is 12.5 times
more soluble in water than is oxygen.
As stated in General Information, ozone is very unstable, which is why is
must be generated at the point of use. It can quickly change back to atmospheric
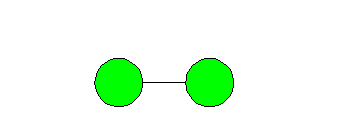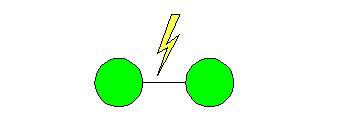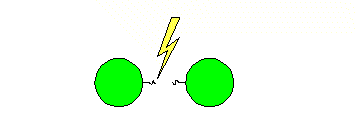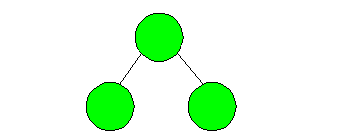
oxygen. Molecular oxygen is composed of two atoms of the element oxygen. Ozone
is comprised of three atoms of oxygen and is formed from molecular oxygen. The
transformation from atmospheric oxygen to ozone is triggered by a high voltage,
low amperage electrical charge (see Generation) or by the addition of
ultraviolet (UV) radiation. The electrical charge breaks the bond between the
oxygen atoms and causes them to form in groups of three as ozone.
Ozone is 12.5 times more soluble in water than is oxygen, leading to better
mixing in water treatment (Towles, 1997, b). Also, the products of its reaction
with organics are oxygen, carbon dioxide, and water. This prevents the
incomplete disinfection products that could lead to trihalomethanes (THMs) in
drinking water. Ozone is effective against odor-producers because it can easily
oxidize these unsaturated compounds.
The reason that ozone is so effective at disinfecting is because of its
oxidation potential, which is -2.07 V. For comparison, the oxidation potentials
of hypochlorite and chlorine are -1.49 V and -1.36 V, respectively (Towles,
1997, b). The only element with a higher value is fluorine (Coate, 1997).
Oxidation potential is important because it indicates the expected degree of
chemical transformation. However, it does not indicate the speed or completeness
of oxidation.
Heat can accelerate oxidation. Another way to raise this rate is by the
addition of ultraviolet (UV) radiation. This combination forms a highly-reactive
hydroxyl ion as the ozone and UV "destroy" each other (Coate, 1997). The free
radicals produced can quickly oxidize a number of organic compounds. Ozone may
not completely oxidize some organic compounds, such as those found in some
industrial wastewaters, but "no other commonly employed and less powerful water
treatment oxidant...will oxidize any organic material completely to carbon
dioxide and water if ozone will not" (Towles, 1997, b).
Although the precise mechanisms of ozone disinfection are not firmly
established, the most acceptable theory is that ozone can disrupt the function
of the bacterial cell membrane by exerting its strong oxidizing capability. It
is an effective disinfectant for a wide range of pathogens and is applicable for
achieving the primary disinfection goal for the pathogen categories regulated in
the EPA Surface Water Treatment Rule (Bryant,et al,1992). 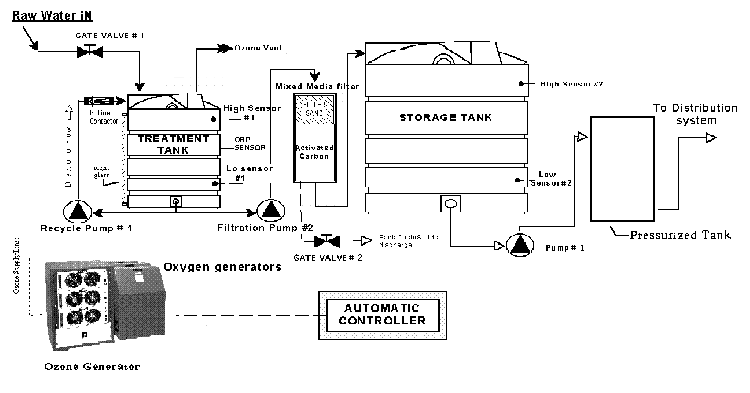 The above
drawing represents a typical ozonating water treatment plant. The above
drawing represents a typical ozonating water treatment plant.
Drawing
courtesy of Ozonair Corporation
Return to
the table of contents

Generation
Ozone can be generated from one of three sources, with varying concentrations
of ozone obtained (Towles, 1997, a):
- Ambient air - 0.15 to 1.0% ozone
- Dried air to a dew point of -40 to -65 C - 1.0 to 3.0% ozone
- Oxygen as feed - 4.0 to 6.0% ozone
As one can surmise, a higher concentration requires more effort and ergo
higher cost. However, the largest expense in an ozone treatment plant is usually
the electricity usage, sometimes accounting for 75% of the cost of running the
plant (EPRI, 1996). Using oxygen as the feed gas will double or triple the
efficiency of ozone production per kWh (Towles, 1997, a).
Whichever source is used, the basic means of production is the same. An
electrical charge of high voltage and low amperage is passed through the gas to
form ozone from oxygen in the corona discharge. Ultraviolet radiation can also
be used to generate ozone. This ozone gas must then be changed to the liquid
phase, which is easy because ozone is 12.5 times more soluble than oxygen. A
decrease in the water temperature correlates with an increase in the solubility
of ozone. 

Advantages/DisadvantagesOzone for Treatment of Water:
| ADVANTAGES |
DISADVANTAGES |
| More powerful disinfectant than chlorine compounds (more effective at
Cryptospiridium removal) |
Tends to cost more than traditional chlorinated disinfection
techniques. |
| Has no negative residuals such as trihalomethane production |
Does not produce a disinfection residual that would prevent bacterial
regrowth. |
| Does not alter the pH of the water |
Forms nitric oxides and nitric acid which could lead to
corrosion |
| Increases coagulation |
|
| Helps with the removal of iron and manganese |
|
| Has taste and odor control properties |
|
Advantages
Ozone has been shown to be the second most powerful oxidizer, after fluorine.
Ozone can disinfect roughly 3000 times faster for Cryptospiridium removal than
can chlorine (DEL Industries, 1997). This allows for either a lower
concentration of the disinfectant or a faster travel time through the treatment
system, whichever is more advantageous to the designer.
Summary of Ct-Value Range for 99% Inactivation of Various Microorganisms by
Disinfectants at 5oC
| Organism |
Free Chlorine
pH 6-7 |
Preformed Chloramine
pH 8-9 |
Chlorine Dioxide
pH 6-7 |
Ozone |
| E.coli |
0.034-0.05 |
95-180 |
0.4-0.75 |
0.02 |
| Pollo I |
0.1-2.5 |
768-3740 |
0.2-6.7 |
0.1-0.2 |
| Rotavirus |
0.01-0.05 |
3806-6476 |
0.2-2.1 |
0.006-0.06 |
| Phage F2 |
0.08-0.18 |
--- |
--- |
--- |
| Giardia Lamblla cysts |
47-150 |
--- |
--- |
0.5-0.6 |
| Giardia muris cysts |
36-630 |
1400 |
7.2-18.5 |
1.8-2.0 |
| Cryptosporidium parvum |
7200 |
7200 |
79 |
5-10 | Source: Inactivation of Microbcarbon
dioxide, and water.
As stated earlier, ozone reacts with almost anything it can. This provides
ozone with strong taste and odor control as well as allowing it to oxidize many
metals and organics. Lab results have shown that ozone can remove the following
metals at 99.5% or above: aluminum, arsenic, cadmium, chromium, iron, nickel,
cobalt, lead, zinc, copper, and manganese (Coate, 1997). Ozone can completely
oxidize mercury at pH 4 as well (Coate, 1997). Ozonation changes nitrite ions to
nitrate ions, and has also been shown to be effective in treating the following:
acetic acid, butoxyethanol, isopropyl alcohol, methyl-ethyl ketone, acetone,
cetyl alcohol, glycerol, propylene glycol, n-butyl acetate, formaldehyde,
methacrylic acid, benzene benzyl alcohol, resorcinol, n-butyl phthalate camphor,
para-phenylenediamine, styrene trecresyl-phosphate, xylene, butane,
liquefied-petroleum-gas, mineral spirits, methylene-chloride, perchloroethylene,
trichloroethylene, hydrogen cyanide, ammonium-hydroxide, ethanolamine, toluene,
isobutane, propane, methyl-chloroform, amino-phenol, ammonia,
ammonium-persulfate-phenacetin, ethylene tetracetic acid (EDTA), alkylated
silicates, and non-ionic detergents (Coate, 1997).
Disadvantages
The primary drawback to the use of ozonation is the cost. Not only are
capital costs significantly higher than its chlorinated alternatives, but the
maintenance and daily operation costs also tend to be higher. Some have
estimated that ozonation can cost up to eight times more than disinfection
(EPRI, 1996). Although this may be true in certain cases if only disinfection is
considered, the total cost benefit will largely depend on the water being
treated. As stated earlier, ozone provides numerous other advantages besides
disinfection: ozone can reduce treatment needed for taste, odor, manganese, and
iron control, and aid in coagulation.
Costs of ozonation by Aquaflow Corporation
| CAPITAL COSTS |
MAINTENANCE COSTS |
| $100,000 per MGD treated |
0.3 kWh per 1000 gallons treated | Costs provided
by the Aquaflow Corporation
Notice that the maintenance costs of ozonation are measured in
kilowatt-hours. This is because the primary cost associated with ozone operation
is the electricity needed to operate the ozone generators. Taking into
consideration the lifetime operation of a treatment plant, some have estimated
that up to 75% of all costs associated with ozonation are due to electricity
needs (EPRI, 1996). Currently, many companies are working on ways to increase
electrical efficiency in ozonators or create methods where less ozone is needed.
Developments in these areas could reduce the cost of ozonation for the
future.
The other major drawback to ozonation is the chance of bacterial regrowth in
the system. Unlike chlorine which leaves a small disinfecting residual
throughout the system, ozone leaves no such residual. Because there is no longer
any disinfecting mechanism to follow the water throughout the distribution
system, there is the possibility that bacteria will begin to regrow in the
water. If this becomes a problem, the solution is to add a small amount of a
chlorinated compound before the water leaves the plant.
Another drawback of ozone is that it can form nitric acid or nitric oxides
which are very corrosive. The solution is to use non-corrosive materials,
non-oxidizable materials in and downstream of the ozone generation and
application system. Return to
the table of contents

Links to Related Sites

References
- Aquaflow Corporation, Web Page, 1997. (http://www.Thomasregister.com/olc/aquaflow/owt.html)
- Bryant, E.A., Fulton, G.P., and Budd, G.C.. Disinfection alternatives for
safe drinking water. Van Nostrand Reinhold, 1992.
- Coate, Robert, The Co-Deca-Tech Water/Wastewater Treatment System, Web
Page, 1997.( http://www.ozonated.com/ptov6-8-.html)
- DEL Industries, Ozone Questions and Answers, Web Page, 1997. (http://206.190.82.21/qaozn.htm)
- Dryden Aqua Ltd, Web Page, 1997. (http://www.ozone.co.uk/disinfection.html)
- EPRI, Ozone Optimization Turns Tide of Concern About Drinking Water
Safety, Web Page, 1996.
- Evans III, F.L., Ozone in water and wastewater treatment. Ann Arber
Science Publishers, 1972.
- Langlais,B. , Reckhow,D.A., and Brink, D.R. Ozone in water treatment.
Lewis Publishers, 1991.
- Towles, John, Ozone Uses in Odor Control, Web Page, 1997, a. (http://www.ozonated.com/ozusinod.html)
- Towles, John, The Technology of Ozone in the Oxidation of Organic
Compounds, Web Page, 1997, b. (http://www.ozonated.com/tufozin.html)

Send comments or suggestions to:
Student Authors: Rob Dunham rdunham@vt.edu, Hong He hhe@vt.edu, and Ken Woodard kwoodard@vt.edu
Faculty Advisor: Daniel
Gallagher, dang@vt.edu
Copyright © 1997
Daniel Gallagher
Last Modified: 2-5-1998 |