
|
|

Disinfection Contact Time and Kineticsby Eric Karch and David LoftisTable of ContentsIntroductionWater is disinfected but never completely sterilized in the water treatment
process. This disinfection is a two part process that includes:
Contact time and kinetics are simply a measure of the inactivation due to time and concentration of the disinfectant. The USEPA has developed regulations for the minimum kill percentages (inactivation) necessary for public water to be considered potable. These regulations include a minimum disinfection of:
Varying degrees of disinfection can be attained by altering the type and concentration of disinfectant, as well as the time water is in contact with the disinfectant. The decision to use one type of disinfectant versus another will set the precedence for the remainder of the values needed to attain the proper disinfection. The time untreated water is exposed to the disinfectant and the concentration of that disinfectant are the main factors in the equation that will be discussed in the next section. (Notice that the units of contact time are (mg/l)(min).) HistoryA relationship between kill efficiency and contact time, was developed by Harriet Chick while she was a Fellow in the Pasteur institute in Paris, France. The research yielded data supporting her relationship that is shown in Figure 2 below. (No) represents the initial number of organisms and N is the number of organisms at time t. As contact time between water and disinfectant increases, the ratio of No/N decreases as Chick's Law predicts. 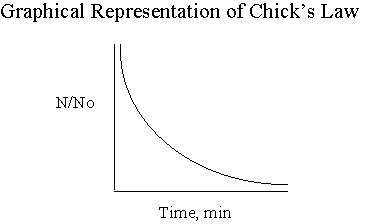 Figure 2 Taken from R.C. Hoehn’s CE 4104 Spring NotesWatson later modified Chick's equation to account for varying types of disinfectants. He developed coefficients that better represented the strength of the disinfectant as well as the pH of the water. From this research, the coefficient of specific lethality (lambda) was developed. Watson’s modification of Chick’s equation is shown below.  Factors Affecting C*t Values
Applications to Real SystemsContact time and credit are essential in the design of a water treatment plant. In addition to the Environmental Protection Agency, the Virginia Department of Health regulates these values. Regulations governing credit have been discussed above, but contact time is dependent upon the specific plant. Most plants attain the required C*T in the clearwell, and the Blacksburg Water Authority (BAT) is no exception. Since its creation in 1950, the water demand in the area has risen sharply. The BAT has been forced to convert all available space to expand the size of the c learwell. The result is a poorly baffled clearwell that does not retain the water long enough to get the credit it needs to meet regulations. The following calculations are not entirely relevant to this topic, but the result is. They are taken from actual calculations by Jennifer Barber for the Blacksburg Water Authority in 1991. Before beginning the calculations, two terms must be defined. T-theo is the time one drop of water spends in the clearwell, theoretically. Baffling factor, BF, is a term that accounts for the mixing that occurs in the clearwell. Short-circuiting can be avoided by proper baffling. Below is a table of BF values. Table 3: Baffling Factor Values
Notes of Jerry Higgins (1991), Blacksburg Water AuthorityThe Blacksburg Water Authority attains credit for disinfection in the clearwell. The volume of the clearwell is 162,760 gallons, and the flow out of the clearwell is 12.4 MGD. T-theo = (162,760 gal) / [ 12.4 MGD*694.4 GPM/MGD ] = 18.9 minBF = 0.4 for BAT T10 = (0.4)(18.9min) = 7.56 min At a pH = 7.8, chlorine residual = 1.0mg/l temperature = 1 degree Celsius log inactivation desired = 0.5 (to supplement the 2.5 credits from using sand filtration) the required C*t = 45 (mg/l)(min) BUT with a T10 = 7.56, the C*t available at BAT = (7.56 min)(1 mg/l) = 7.56 (mg/l)(min) This corresponds to a log inactivation available of 0.084. This is well below the desired credit of 0.5. The Blacksburg Water Authority is investigating possible solutions to this problem. Adjusting the pH with the use of caustic (as shown in Figure 4) could increase the effectiveness of the disinfectant. This would increase the concentration of (HOCl), which is more efficient and powerful than its counterpart, (OCl-). Figure 4: Blacksburg Water Authority Caustic Addition System 
The most likely solution, though, will involve the construction of an on-site storage facility. This will increase the time water is in contact with the chlorine, thus increasing the log inactivation and credit for disinfection. Credits and LinksWe would like to thank those who made this project possible. Dr. Hoehn and
Dr. Gallagher of Virginia Tech provided us with technical information and
computer assistance. Jerry Higgins of the Blacksburg Water Authority took the
time to show us how this topic applied to his water treatment plant. For more
information regarding Blacksburg's water supply, visit their web site at
Send comments or suggestions to: Faculty Advisor: Daniel Gallagher, dang@vt.edu Copyright © 1997 Daniel Gallagher Last Modified: 2/24/1998 |