Disinfection
with Ozone Disinfection
with Ozone 
by Stacie Kramer and Susanna Leung
Table of Contents: Definition
Definition
 Background
Background
 The
Ozone Journey The
Ozone Journey
 Evaluation of Ozone as a Disinfectant
Evaluation of Ozone as a Disinfectant
 References
References
Definition:
Ozone is a form of oxygen with the molecular formula O3. It forms
when O2 or clean dry air is exposed to a powerful electric current.
In nature, it forms in the upper atmosphere when lightning passes through the
air. Ozone is unstable and changes to O2 shortly after its formation.
It is a powerful oxidant and one of the most powerful disinfectants available in
water treatment.
Background:
Ozone was discovered in 1783, by VanMarum but was not named until 1840. In
1857, Siemens developed the first electric discharge ozone generating device and
by 1893, it was utilized for potable water disinfection in Oudshoorn,
Netherlands. Ozone began gaining popularity in Europe as people realized its
potential for disinfection and other uses. Since then, it has become a widely
used disinfectant in all parts of Europe. The water treatment plant in Nice,
France has continued to use ozone for disinfection since 1906.
Only recently
has the use of ozone gained national attention in the United States. Although
New York City's Jerome Park Reservoir began applying ozone in its treatment of
water for taste and odor control since 1906, there were only 5 water treatment
facilities using ozone in the United States in 1987 (3).
In the past, America has relied more heavily on less expensive disinfectants
such as free chlorine, chlorine dioxide, and chloramines. Recent legislature,
such as the Safe Drinking Water Act Amendments, Surface Water Treatment Rule and
other proposed bills place stricter rules on both the range and amount of
disinfection needed and the concentrations of disinfection-by-products allowed
in drinking water. Therefore, the use of free chlorine and other common
disinfectants are becoming less cost-effective. Ozone is one of the few
disinfectants that can effectively inactivate both Cryptosporidium and
Giardia
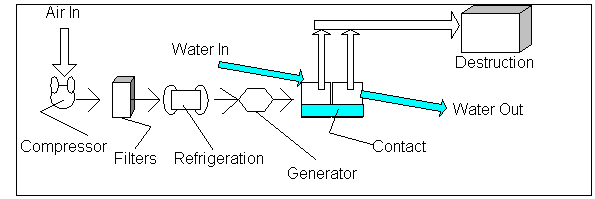
Flow path of ozone in water treatment
The Ozone Journey:
Gas Preparation
Click to download animation
Ozone is a highly unstable molecule with a relatively short
half-life. It must therefore be generated on-site. Ozone can be generated from
any source of gas which contains oxygen molecules. The most common sources for
ozone generation are oxygen gas tanks or air in the atmosphere. Although the use
of pure oxygen gas will result in a higher efficiency of ozone generation, it
will also increase the initial cost of the gas source. On the other hand, using
the atmosphere requires preparation of the gas source. The atmosphere must first
be compressed, cleansed and dehumidified (3).
Compression of the air serves to increase the concentration of ozone in the
supply. The removal of foreign particulate such as dirt and dust is often
accomplished through the use of filters. Air humidity is decreased usually by
lowering the dew point by refrigeration. Most ozone generators require clean,
dry gas for optimal conditions.
Generation
Click to download animation
The clean, dry gas passes through a chamber with a high voltage
electric current which discharges electrons into the air. The electrons convert
the oxygen molecules to ozone molecules and oxygen atoms. The highly unstable
oxygen atoms bond with hydrogen atoms in the air to form hydroxyl radicals. The
gas is then injected in the water passing through the system and solubilized.
The solubility of ozone in water has been found to obey Henry's Law and is a
function of temperature and pH (3).
The amount of ozone in the system can be regulated in the generator by adjusting
the voltage of the current or the flowrate of the gas. The maximum ozone
concentration produced by a generator is 50 g/m3 and the maximum
solubility concentration of ozone in water is 40 mg/L (3).
Contact
Click to download animation
The ozone-enriched water flows into a chamber to allow for sufficient contact
time. During this contact time, ozone molecules decay to form oxygen molecules
and free hydroxyl radicals. It is both the ozone and the highly reactive free
radicals which oxidize organic molecules. Through this oxidation, toxic
herbicides and pesticides are reduced to more environmentally friendly
components (6).
Non-biodegradable organics will reduce to smaller biodegradable parts. The
reaction is capable of splitting proteins, carbohydrates and humic acids at
double-bonded carbons to damage and destroy critical components of organisms
found in the water. Since the free hydroxyl is so highly reactive, the contact
time necessary is minimal, as compared to other disinfectants (6).
Destruction
Click to download animation
Ozone has an average half-life of 20 minutes if it is not oxidized
by particulates (5).
Depending on the water quality at a given time, a portion of the dissolved ozone
may pass through the system unoxidized. Ingestion of ozone is a serious concern
because of its strong oxidizing capabilities. Safety precautions are usually
taken to insure that the remaining ozone molecules are destroyed before they
reach the distribution system. Regulations require the resulting concentration
of ozone to be reduced to less than 0.002 mg/L before the water is released from
the plant (6).
Ozone degradation can be accelerated by the addition of hydrogen peroxide or
passing the system through a UVc system. Contact time can also be lengthened to
allow for further destruction.
Evaluation of Ozone as a Disinfectant:
| Pros |
Cons |
| - Extremely powerful disinfectant |
- Expensive option |
| - Does not form trihalomethanes |
- Can form other hazardous
disinfection-by-products such
as bromate |
- Requires relatively short
contact time |
- Requires high level of
technology |
| - Reduces taste, odor, and color in water by oxidizing the
algea and humic material which causes these problems (2)
|
- Requires another disinfectant to achieve residual
disinfection levels (1) |
| - Forms microfloc upon contact therefore improving
coagulation and reducing the required coagulant dose (2) |
- Unstable - must be generated
on-site |
| - Can improve filtration rates. With improved coagulation,
more material settles in the sedimentation basin. Hence, less material
reaches the filters and the filters can be run longer before backwashing.
(2) |
- Climate control needed to
maintain solubility |
- Environmentally friendly -
decays back to oxygen |
- Not widely used in US (1) |
References:
(1) American Water Works Association Research Foundation. "The
Ozone Option". 1986.
(2) Asea Brown Boveri. "The Use of Ozone in Drinking
Water Treatment"
(3) Haas, Charles N. "Disinfection". Water Quality and
Treatment. Ed. Fredrick W. Pontius. 4th ed. AWWA McGraw-Hill Inc.: New York,
1990.
(4) Reynolds, Tom D., and Richards, Paul A. Unit Operations and
Processes in Environmental Engineering. PWS: Boston, 1996.
(5)
http://ies-energy,com/sustomer_programs/new_technologies/ozonation.html
(6)
http://www.drydenaqua.com/Ozonation/ozonation.htm
Send comments or suggestions to:
Student Authors: Stacie Kramer skramer@vt.edu and Susanna Leung sleung@vt.edu
Faculty Advisor: Daniel
Gallagher, dang@vt.edu
Copyright © 1997
Daniel Gallagher
Last Modified: 2-24-1998 |